- Nitrification is the biological conversion of ammonium (NH4+) to nitrate (NO3-)
- Nitrate nitrogen is subject to losses due to leaching and denitrification
- Nitrification inhibitors slow the biological conversion to nitrate and prevent N loss
The Nitrification Process
Nitrification is the biologically-mediated conversion of ammonium (NH4+) to nitrite (NO2-), followed by the subsequent oxidation of nitrite to nitrate (NO3-). The chemical equation for this two-stage soil biological process is as follows:
2NH4+ + 3O2 -----> 2NO2- + 2H2O + 4H+ + energy (mediated by bacteria in genus Nitrosomonas)
2NO2- + O2 -----> 2NO3- + energy (mediated by bacteria in genus Nitrobacter)
The process is relatively fast, with conversion proceeding in a matter of days to weeks from the point of addition of an ammonium fertilizer. The rate of the reaction depends on soil temperature, presence of oxygen, water content, pH, and fertilizer source.
The biological process is highly temperature dependent, slowing to negligible levels at soil temperatures below 50 degrees F, and no longer limited at temperatures greater than 75 degrees F (see Image 1).
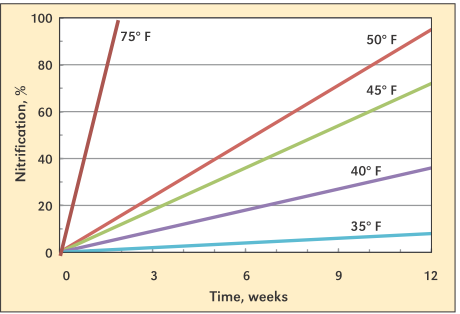
Nitrification is an aerobic process, meaning that oxygen is required. Nitrification rates become restricted when water-filled pore space exceeds 60%, such as in the first couple days after a saturating rainfall event. Rates quickly resume after free water drains from the soil profile and oxygen is restored. Nitrification rates can also be limited by a lack of water
Note also that the first step releases free hydrogen ions, which acidify the soil and lower soil pH, necessitating the monitoring of soil pH and possible limestone additions. Nitrification rate is optimal when soil pH falls between 6.5 and 8.0. Rates slow in acidic soil, but limestone additions will quickly restore microbial activity.
The source of ammonium may influence the rate of denitrification. Localized high concentrations of ammonia can inhibit nitrifying bacteria, such as with anhydrous ammonia application. Anhydrous ammonia can halt nitrification activity for a period of 3-8 weeks, until nitrifying bacterial populations can recover and commence the process.
Nitrification Inhibitors
Nitrate is vulnerable to leaching (coarsely textured soils) and denitrification losses (in finely textured soils). These losses may be as much as 35% of total applied N, depending on environmental factors during the season. A nitrification inhibitor may be used to control the rate of nitrification and keep nitrogen temporarily in the ammonium form to prevent N losses from the cropping system. Although several products are labeled as nitrification inhibitors, the most common active ingredient and one that has been scientifically proven to inhibit nitrification activity is nitrapyrin [2-chloro-6-(trichloromethyl) pyridine].
Nitrapyrin acts as a specific bactericide to Nitrosomonas, with activity lasting 6-8 weeks in cool spring soils and up to 30 weeks with fall applications. In warmer soils, nitrapyrin can degrade in as little as 30 days. Nitrapyrin is the active ingredient found in N-Serve 24 and Instinct II (both from Dow).
N-Serve is an oil-soluble product designed primarily for use with anhydrous ammonia. While the product may be used to stabilize other liquid and dry ammonia fertilizer sources, keep in mind that nitrapyrin is volatile and must be incorporated immediately to minimize product loss. N-Serve is applied at a rate of 32 fl oz per acre.
Instinct II is a water-based microencapsulated formulation of nitrapyrin and is designed primarily for use with liquid ammoniacal fertilizer products, such as UAN and manure. Instinct II is applied at rates of 37 to 74 fl oz per acre.
Considerations for Use
Nitrification inhibitors can be an excellent tool in the 4R toolbox to manage production risk and reduce nutrient losses from agricultural systems to the environment. Fall applications of anhydrous ammonia should be made after soil temperatures fall below 50 degrees F and should always include a nitrification inhibitor to hedge against variable weather conditions. Early spring applications of anhydrous ammonia or UAN may benefit from nitrification inhibitors in tile-drained systems and sandy soils (with greater leaching loss potential) or soils with poor internal drainage (with greater denitrification loss potential). Nitrification inhibitors act as insurance against loss and allow farmers to safely and confidently reduce nitrogen application rates to levels recommended by the Maximum Return To Nitrogen (MRTN) method.
Contact your FS Crop Specialist for your agronomic needs.
Image 1. Effect of temperature on nitrification rates. (Source: IPNI)